The ancient Mesopotamians may not have known what caused their dogs to lash out and seize, but they knew that if one of these dogs bit a human, the results would be deadly. The first written record of rabies dates back to 1930 BCE in the Mesopotamian Laws of Eshnunna, but it likely existed long before that (1).
“We've known about it as long as there have been human stories,” said Erica Ollmann Saphire, a structural biologist and infectious disease researcher at the La Jolla Institute for Immunology.

While the Mesopotamians realized that rabies came from a dog’s saliva, it took another 3,700 years to prove it. In 1804, German physician Georg Gottfried Zinke showed that injecting saliva from a rabid dog transferred the infection to a healthy dog (2). Louis Pasteur and his colleagues at the École Normale Supérieure found that if they transferred the saliva from a rabid dog to a monkey and then from that monkey to another one, the virus weakened with each new transfer. Using that attenuated rabies virus, Pasteur developed the very first vaccine for rabies in 1885 (2).
Nowadays, there is an inactivated rabies virus vaccine for people at high risk for contracting rabies. But while effective, the vaccine does not give long lasting immunity. People living at risk for contracting rabies need to get regular booster shots every three years or so (3).
If people who have not been vaccinated get bitten by a suspected rabid animal, postexposure prophylaxis (PEP) is an effective treatment if taken soon after the bite. PEP consists of both the rabies vaccine and human rabies immunoglobulins to neutralize the virus before the vaccine takes effect. But once rabies symptoms begin, the disease is practically 100 percent fatal. At that point in the infection, there is no treatment.
In wealthy nations like the United States, rabies cases from dog bites are rare, but about 50,000 people require PEP treatment for encounters with bats, skunks, raccoons, and other wild animals (4). The Centers for Disease Control and Prevention (CDC) estimates that the US spends anywhere from $245 to $510 million every year on rabies prevention and treatment. In countries with fewer resources, the outlook is bleaker. Many cannot afford PEP.
In the Kenyan setting where I work, we've had five children this year — in fact, within the last six months — come through with rabies.
- Darryn Knobel, Ross University
“The rabies immunoglobulin, which is a component of PEP, is also the most expensive part because it's made from human or equine” immunoglobulins, said Todd Smith, an infectious disease researcher at the CDC. “A lot of times they'll receive either the equine product… or they will just receive vaccine with no passive antibody to protect.”
With more than 3 billion people living at risk for rabies infections worldwide and approximately 50,000 to 60,000 people dying from the infection every year, better and more accessible preventative care and treatments are still needed (5).
“In the Kenyan setting where I work, we've had five children this year — in fact, within the last six months — come through with rabies,” said Darryn Knobel, a rabies researcher at Ross University.
Stymied by a lack of funding and interest, new rabies treatments and improved vaccines have stagnated. But recent achievements in mapping the full structure of the rabies glycoprotein and advances in neutralizing monoclonal antibody cocktails are bringing potential new rabies therapies and vaccines to the forefront, giving hope for making rabies a completely treatable infection.
A sneaky killer
Despite being a tiny virus with only five proteins at its disposal, rabies does a lot of damage. The rabies virus is a member of the lyssavirus genus — lyssa meaning “rage” or “fury” in Greek — a group of bullet-shaped viruses that preferentially infect neurons.
When an animal infected with rabies bites a human or another animal, the rabies virus typically enters the host’s neurons at a neuromuscular junction at the bite site. Rabies only expresses one protein on its surface: its glycoprotein. With that single protein, it binds to a number of different cellular receptors to make its way into neurons.
This “is one of the reasons why it's so hard to combat and develop a good therapy because it has lots of ways of getting into a cell,” said Maegan Weltzin, an ion channel physiologist at the University of Alaska Fairbanks who studies the interaction between rabies virus and nicotinic receptors.
Once inside a neuron, the virus travels through the host’s peripheral neurons, making its way to the brain (6). For this reason, rabies bites to the face are more serious than bites to the legs. The neurons in the face are much closer to the brain, so the virus can get to the brain faster. While rabies travels through the peripheral nerves, infected patients may have some mild flu-like symptoms and pain at the bite site. At this point, PEP treatment is likely still effective.
Surprisingly, the immune system doesn’t mount a strong response to the virus while it’s in the peripheral nervous system. Some researchers hypothesize that this may be because the rabies virus glycoprotein is structurally heterogeneous, perhaps making it difficult for the immune system to generate effective neutralizing antibodies.
Once the virus reaches the brain, all bets are off. As it begins to replicate in the brain, it causes neuronal dysfunction, and the classical clinical rabies symptoms emerge: agitation, anxiety, hyperactivity, difficulty swallowing, fear associated with swallowing water, and hallucinations among others.
Once patients reach this stage, the only therapeutic option is to keep them comfortable. There have been some anecdotal reports of patients recovering from this kind of clinical rabies infection by being placed in a coma and treated with the rabies vaccine, rabies immunoglobins, antivirals, and ketamine (7). But with such a high failure rate, many clinicians recommend against this treatment avenue.
Although the virus wreaks havoc inside the brain, the overall brain structure looks normal.

“The virus really needs intact neuronal architecture in order to spread trans-synaptically, so it's going out of its way, at least from an evolutionary perspective, to avoid triggering that immune system that could actually cause that kind of damage,” said Knobel. “It's one of the reasons why I have some optimism for a treatment because, yes, definitely some degeneration or dysfunction has been triggered. But by and large, the architecture remains intact.”
Knobel and his team hypothesize that a rabies viral infection is essentially “an extremely acute, rapidly progressive neurodegenerative disease,” he said. Using that idea as a starting point, he and his collaborators are looking for neurodegenerative disease biomarkers in dog rabies patients to see if they can shed light on rabies disease processes. They have begun examining infected neurons in vitro and hope to advance their studies into animal models in the future.
“Really getting to understand what is going on in those neurons, I think is a key area of this translational kind of research,” said Knobel.
Silver bullet antivirals and monoclonals
A good rabies treatment will catch the virus before it reaches to the brain. Researchers are exploring antiviral and monoclonal antibody therapies. So far, there are no antivirals that work against rabies, but that hasn’t stopped Smith and his team at the CDC from looking.
“We routinely screen antiviral compounds and drugs that we hear about or that we're working on for other viruses,” said Smith. Recently, he and his team came across a proprietary form of the antiviral ranpirnase called TMR-001 (8). The antiviral seemed to be effective against poxviruses, so Smith and his team decided to see how it fared against rabies virus.
“It worked really well in cell culture, but it did not protect animals, which is not uncommon for rabies antivirals,” said Smith. “Part of that is just the problem of drug delivery. Getting the drug to the right place at the right time and having it be active enough to slow down and stop the virus… is really difficult once that cascade in the central nervous system starts.”
That's the silver bullet that everybody in the rabies field is always looking for — the treatment piece of it.
- Todd Smith, Centers for Disease Control and Prevention
Smith and his team haven’t given up hope on an antiviral strategy. They are looking for other antivirals to test against rabies and for approved drugs that may also have antirabies effects.
“That's the silver bullet that everybody in the rabies field is always looking for — the treatment piece of it,” said Smith. For now, he and his team have shifted their focus to improving PEP with monoclonal antibodies. The human rabies immunoglobulins in PEP consist of a mixture of antibodies that a person’s immune system produces in response to rabies vaccination.
“Monoclonal antibodies have been proposed a long time ago, probably 30 years ago, as a potential candidate to replace the rabies immunoglobulin,” said Smith. “The idea is that a cocktail of monoclonal antibodies could be produced on a larger scale and cheaper than the current immunoglobulin product,” which requires human donors.
So far, the only approved monoclonal antibody product for rabies is in India where the main source of rabies infections is dogs. In the United States, where rabies infections typically come from bats, a single monoclonal antibody likely would not provide sufficient treatment because bats typically carry many different rabies virus variants. Instead, a monoclonal antibody cocktail could work.
Smith and his team recently collaborated with researchers at MassBiologics of the University of Massachusetts Chan Medical School to identify a monoclonal antibody cocktail against the main strains of rabies virus found in North America (9). The first antibody cocktail they tested had some trouble neutralizing some of the strains, but when they switched to their second mix, it neutralized all of the rabies variants tested.
“That's what we always hope,” Smith said. Moving forward, Smith and his team have additional ongoing collaborations testing other monoclonal antibody cocktails against these rabies variants.
A glycoprotein breakthrough
The biggest hurdle facing the development of better rabies vaccines and therapies has been that no one really knows what the rabies surface glycoprotein looks like.
“Even when I was a graduate student in the early 90s, people were trying to get the structure of the surface protein of rabies because of its fundamental importance to human health and to animal health, and it hadn't yet been possible,” said Saphire. “It's shocking given how fundamentally important rabies is to human and animal health, that we know so little about how it interacts with its receptors and what the human immune response might be.”
Because of the heterogeneity and flexibility of the rabies glycoprotein, prior attempts to determine its structure required researchers to modify the protein to crystalize it. In a recent attempt, researchers mapped the monomer of the glycoprotein, but they had to chop off flexible pieces of the glycoprotein called the fusion loops to map it, meaning that a lot of structural information about the trimeric protein was still missing (10).
“Sometimes the most potent antibodies are those that bridge different pieces of the glycoprotein together. They bridge the subunits, or they bridge the different monomers and the trimer,” said Saphire. “To evaluate what those most potent and most protective kinds of antibodies might be, we needed to understand exactly how all the different domains and different monomers get gathered together in that final structure.”
The key hurdle that Saphire and Heather Calloway, the postdoctoral fellow in her laboratory who led the project, needed to surmount was how to stabilize the glycoprotein’s flexible fusion loops in their natural conformations.

First, Callaway and her colleagues bound the glycoprotein with a neutralizing antibody, which locked the rabies glycoprotein in its prefusion form — the form it takes before it infects a cell. With the glycoprotein locked in place, Callaway isolated it in a detergent to keep the protein in its native state. She and her team then took advantage of cryo-electron microscopy’s (cryoEM) unique capability to capture flexible and heterogeneous structures to solve the glycoprotein’s prefusion structure (11).
“You see within the cryoEM that it does have multiple conformations, so if you put them all together, you can see sort of a motion. It's almost like the fusion loops are dancing,” said Callaway.
Unlike other viral glycoproteins, which typically keep their fusion loops tucked on the inside of the proteins, the rabies virus glycoprotein’s fusion loops gather at its base to help stabilize the protein in its trimeric form. “This explains why that structure had been so elusive for so long,” Saphire added.
After imaging at least 100,000 rabies virus glycoprotein particles over three years, Callaway, Saphire, and their team finally revealed the roadmap to better rabies vaccines and antibody therapies. With a clear and complete structure, they and other researchers can study where other neutralizing antibodies bind and engineer antibodies that bind the rabies prefusion glycoprotein better.
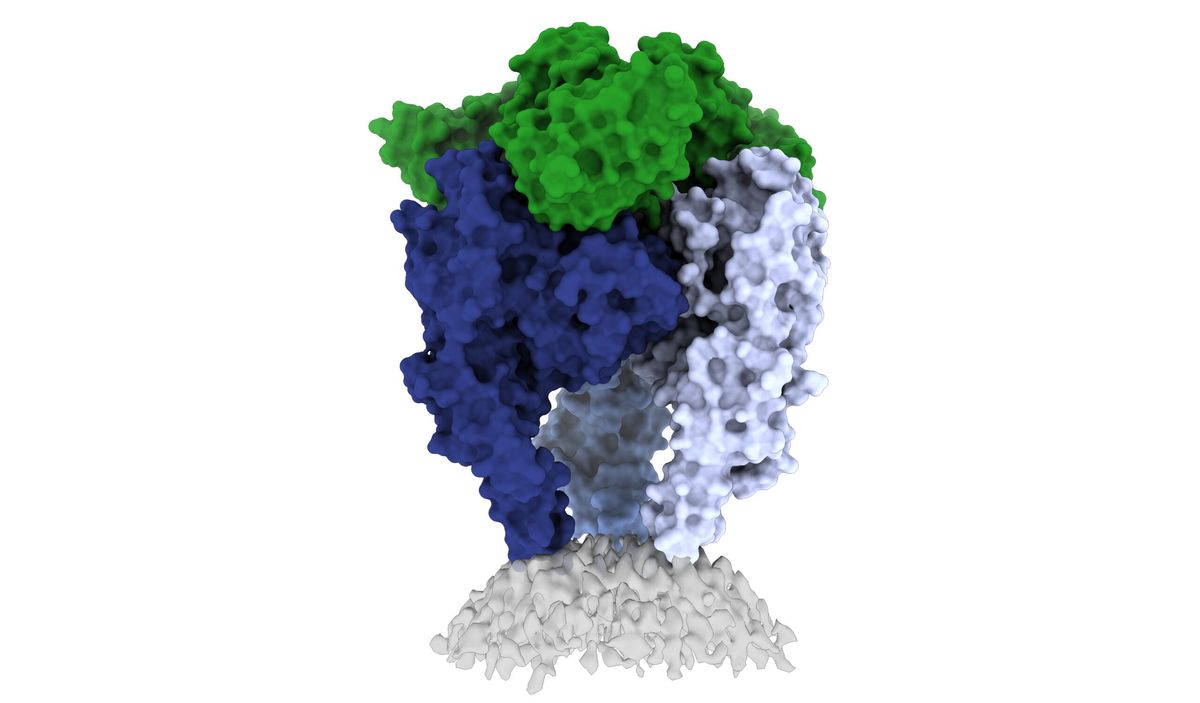
Saphire and her team are now studying the structure of the rabies glycoprotein when bound to different antibodies. They’re also designing more precisely engineered vaccines for other lyssaviruses that look like rabies but do not respond to rabies vaccines or PEP treatments such as the Australian bat lyssavirus (ABLV) and European bat lyssavirus (EBLV), which cause symptoms identical to rabies in humans (12).
“Many of them exist in bats and can infect humans,” said Callaway. “Have they gotten out of bats and into other carnivore populations like rabies? Not yet, but they could. And if they did, we would like to have vaccines or antiviral antibodies already ready to combat this kind of potential pandemic.”
With increased human encroachment on bat ecosystems, the potential for bat and human interactions increases. More rabies researchers such as Matthias Schnell at Thomas Jefferson University are developing vaccines against rabies-related viruses. He uses a deactivated rabies virus as a vaccine platform, and he and his team are creating new vaccines based on the antigenic regions of Mokola lyssavirus and Lagos bat lyssavirus.
“It's really hard to start from zero,” said Schnell. “You really don't know when you will need it.”
Daring small molecules
With new antivirals, monoclonal antibodies, and vaccines all in the works, researchers at the drug discovery company Prosetta Biosciences take a unique approach to rabies therapy: small molecules.
Like all viruses, rabies virus hijacks host proteins to replicate. Vishwanath Lingappa, a molecular biologist and leader of Prosetta Biosciences, realized that instead of targeting the virus, they could make an antiviral to take back the hijacked host proteins.
In earlier research, Lingappa’s colleagues discovered that host proteins form transient multiprotein complexes to help assemble viral capsids (13). Lingappa and his colleagues reasoned that if they found a small molecule that impaired the host multiprotein complex that the viruses use to replicate and assemble, they could interfere with viral assembly. But their path to searching for rabies antivirals was as unconventional as their approach.

“We did it actually almost on a dare,” said Lingappa. As he explained his small molecule antiviral approach to a virologist colleague, “he said, ‘no, there are no small molecules against rabies.’ I said, ‘Well, we can find small molecules for any viral family.’ He wouldn't believe it, and I said, ‘Okay, you've pissed me off, so I'm going to find them.’”
To find small molecules that bound and impaired host proteins that aid rabies virus assembly, Lingappa took advantage of his expertise in cell-free translation. Using Prosetta Biosciences’ library of small molecules, he and his team screened for molecules that interfered with rabies virus capsid formation in a cell-free translation assay (14). They found that a molecule called PAV-866 targeted a portion of the host cell’s supply of the protein ABCE1 and blocked rabies virus assembly in vitro.
“Of all the ABCE1 in the cell, only about a few percent are in the multiprotein complex that rabies needs. The other 95 plus percent are doing other things,” said Lingappa. Because of that, an approach like CRISPR or siRNA knockdown won’t solve the problem of only inhibiting the subpopulation of the protein that the virus needs.
We've almost convinced ourselves that rabies is untreatable and always will be. But I think it's really time to revisit that and really try and imagine rabies as a treatable disease and do the kind of research and development that will bring us there.
- Darryn Knobel, Ross University
In their recent work, Lingappa and his team figured out that to co-opt host machinery, viruses bind to an allosteric site on the target protein to help it form a capsid. By binding to the protein’s allosteric site, “the virus tweaks it this way. Our small molecule tweaks it that way,” impeding viral assembly in the process, said Lingappa. Their most advanced candidate based on this mechanism is a SARS-CoV-2 small molecule antiviral (15).
Since their in vitro success with PAV-866 and other small molecules that inhibit rabies virus assembly, the team has pushed these candidates even further.
“We sent them to the CDC. They said, 'oh my gosh, these work,'” said Lingappa. That finding “was our first identifying of a small molecule that was efficacious in cell culture. We've subsequently advanced that. I have a molecule that shows efficacy in mice infected with rabies, even when you wait until the animals have rabies in the brain,” he added. “It wasn't complete efficacy, but it was certainly a great start.”
Lingappa plans to begin studies for this rabies therapeutic and to one day get them into human clinical trials, but like many researchers working on rabies, he hopes to first find funding to get the studies up and running.
“We've almost convinced ourselves that rabies is untreatable and always will be,” said Knobel. “But I think it's really time to revisit that and really try and imagine rabies as a treatable disease and do the kind of research and development that will bring us there.”
References
- Tarantola, A. Four Thousand Years of Concepts Relating to Rabies in Animals and Humans, Its Prevention and Its Cure. Trop Med Infect Dis 2, 5 (2017).
- Pearce, J.M.S. Louis Pasteur and Rabies: a brief note. Neurol Neurosurg Psychiatry 73, 82 (2002).
- CDC. Pre-exposure Prophylaxis (PrEP). (2022). https://www.cdc.gov/rabies/prevention/pre-exposure_vaccinations.html
- CDC. Cost of Rabies Prevention. (2019). https://www.cdc.gov/rabies/location/usa/cost.html
- Hampson, K. et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 9, e0003709 (2015).
- Begeman, L. et al. Comparative pathogenesis of rabies in bats and carnivores, and implications for spillover to humans. Lancet Infect Dis 18, e147-e159 (2018).
- Willoughby, R.E. et al. Survival after Treatment of Rabies with Induction of Coma. N Engl J Med 352, 2508-2514 (2005).
- Smith, T.G. et al. Antiviral Ranpirnase TMR-001 Inhibits Rabies Virus Release and Cell-to-Cell Infection In Vitro. Viruses 12, 177 (2020).
- Ejemel, M. et al. A cocktail of human monoclonal antibodies broadly neutralizes North American rabies virus variants as a promising candidate for rabies post-exposure prophylaxis. Sci Rep 12, 9403 (2022).
- Yang, F. et al. Structural Analysis of Rabies Virus Glycoprotein Reveals pH-Dependent Conformational Changes and Interactions with a Neutralizing Antibody. Cell Host & Microbe 27, 441-453.e7 (2020).
- Callaway, H.M. et al. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Sci Adv 8, eabp9151 (2022).
- Warrell, M.J. & Warrell, D.A. Rabies and other lyssavirus diseases. The Lancet 363, 959-969 (2004).
- Lingappa, J.R. et al. A Multistep, ATP-dependent Pathway for Assembly of Human Immunodeficiency Virus Capsids in a Cell-free System. J Cell Biol 136, 567-81 (1997).
- Lingappa, U.F. et al. Host-rabies virus protein-protein interactions as druggable antiviral targets. Proc Natl Acad Sci U S A 110, E861-8 (2013).
- Müller-Schiffmann, A. et al. A Pan-respiratory Antiviral Chemotype Targeting a Transient Host Multiprotein Complex. Preprint at: https://www.biorxiv.org/content/10.1101/2021.01.17.426875v3



