Deep in the rural countryside about 300 miles north of Mexico City, there is a small town called Ciudad Mante. Known for harvesting and producing sugarcane, Ciudad Mante’s motto is “Donde el azúcar es más dulce que la miel,” the place where sugar is sweeter than honey. The motto, while charming, belies the implications of a fruitful industry.
“In Mexico, there's a huge epidemic of obesity, and that leads to a lot of severe metabolic diseases, including diabetes,” said Emma Lessieur, a postdoctoral scholar studying diabetic retinopathy at the University of California, Irvine who grew up in Ciudad Mante. “A high percentage of the population in my small town has diabetes, and blindness is a common complication that I see in patients in my community. Since I was growing up, I was always intrigued about diabetic retinopathy and ways to prevent it.”

Diabetic retinopathy is a condition where blood vessels in the retina are damaged due to the high blood sugar levels in diabetes. The condition affects more than one third of the approximately 422 million people living with diabetes worldwide (1).
High blood glucose levels damage blood vessels throughout the body, which is why diabetes can lead to kidney failure, neuropathy, and foot ulcers. But certain parts of the body such as the retina are particularly sensitive to blood vessel damage.
“The retina requires a good vascular supply because the ability to sense light and to be able to see things actually requires a lot of energy,” said Leo Kim, an ophthalmology researcher and clinician at Harvard Medical School.
Damaged blood vessels leak. Blood seeping from retinal blood vessels into the back of the eye triggers swelling and inflammation. These damaged blood vessels also don’t carry blood to the retina as well as they should, which can cause ischemia or low oxygen levels there.
“The retina realizes it's ischemic — it's not getting enough blood — so it sends out a signal to create more blood vessels,” said Kim. “The problem is that those blood vessels that do come around are not normal.” Because the vessels shouldn’t be there in the first place, there’s not enough room to accommodate them all. This leads to the formation of scar tissue in the back of the eye. The inflammation, leaky vessels, lack of oxygen, growth of new blood vessels, and scar tissue formation all contribute to vision loss.
Luckily, treatments for diabetic retinopathy exist. The most commonly prescribed ones are ranibizumab (brand name: Lucentis) and aflibercept (brand name: Eylea). These drugs target proteins called vascular endothelial growth factors (VEGF), which promote blood vessel growth. These VEGF inhibitors reduce blood vessel leakiness and block the signal from the ischemic blood vessels that creates more blood vessels.
The problem is that because these VEGF inhibitors are protein-based drugs, they are too big to diffuse through the different layers of the eye to get to the retina if applied topically, and no oral formulations of these drugs exist. They must be injected directly into the eye as often as every four to eight weeks for the rest of a person’s life.
“Patient compliance is very poor,” said David Kita, who leads the scientific direction at the biotechnology company Verseon, which is developing a treatment for diabetic retinopathy. Even with the regular injections, “many of the people don't see any significant improvement, but they're desperate. So, what else are they going to do at that point?”
VEGF inhibitors are also expensive. In 2020, the United States government spent more than $4 billion covering aflibercept and ranibizumab alone in the Medicare Part B budget (2).
People with diabetic retinopathy need more affordable and less invasive treatment options. Through the identification of new drug targets and innovative delivery methods, scientists are developing both eye drops and oral drugs to treat diabetic vision loss without the need for eye injections.
Drops of small molecules
When diabetic retinopathy is in its early stages, most people can see just fine. But as it advances to later stages, the consequences quickly become serious. In his clinical work, Kim often sees patients with proliferative diabetic retinopathy, one of the most severe forms of the disease.

“It's the worst form because you have to operate on many of these patients,” he said. “They get bleeding inside the eye, or they get retinal detachments associated with the formation of fibrovascular membranes — this scar tissue that forms inside the eye.”
To try to understand what was going on in these patients’ eyes, Kim and his team began collecting samples from his patients’ eyes after performing surgeries. After sequencing the transcriptomes of the samples, the researchers noticed that the gene for Runt-related transcription factor 1 (RUNX1) was overexpressed in patient tissue samples compared to control healthy samples (3).
Cancer researchers also know RUNX1 by the name AML1 due to its importance in acute myeloid leukemias (4). Increased RUNX1 expression leads to increased blood vessel formation, which tumors often use to increase their blood supply. Because of its importance in cancer, scientists had already developed many different RUNX1 small molecule inhibitors. Kim and his team eagerly investigated these inhibitors in relieving the increased blood vessel formation seen in diabetic retinopathy.
They started by injecting a small molecule RUNX1 inhibitor called Ro5-3335 into mouse eyes (3), but they soon realized that they could formulate Ro5-3335 into a nanoparticle emulsion. They encapsulated Ro5-3335, which has a high affinity for lipids, in oil-phase molecules and mixed those particles in a saline solution. Using this formulation, the researchers applied Ro5-3335 directly to the surface of the eye as eye drops (5). When they treated both mouse and rabbit eyes with the drops, the RUNX1 inhibitor had no problem reaching the back of the eye at the needed therapeutic concentration.
“We're all just happy that we're able to get it back in there,” said Kim. “If the drug is able to get back there, then it's likely to be able to be effective.”
Both RUNX1 and VEGF inhibitors block new blood vessel formation, but only RUNX1 blocks scar tissue formation in the eye (5). When the COVID-19 pandemic shut down laboratories around the world in the spring of 2020, Kim wondered if RUNX1 inhibitors could affect lung fibrosis due to the novel SARS-CoV-2 viral infection. He and his team discovered that SARS-CoV-2 infection increased RUNX1 expression in patient lungs compared to healthy ones and that RUNX1 inhibitors could prevent the activation of fibrosis in vitro (6).
Multiple RUNX1 inhibitors are working their way through clinical trials in the context of treating various cancers, and Kim is interested in testing RUNX1 inhibitors in non-human primate eyes for their role in treating diabetic retinopathy.
“Ten years from now, somebody could be just using drops to manage their diabetic retinopathy,” he said. “It would change their lives.”
Cellular text messages
While Kim focuses on stopping new blood vessel and scar tissue formation, Lessieur has plans for another eye drop solution for diabetic retinopathy. But she’s targeting a different hallmark of the disease: inflammation.
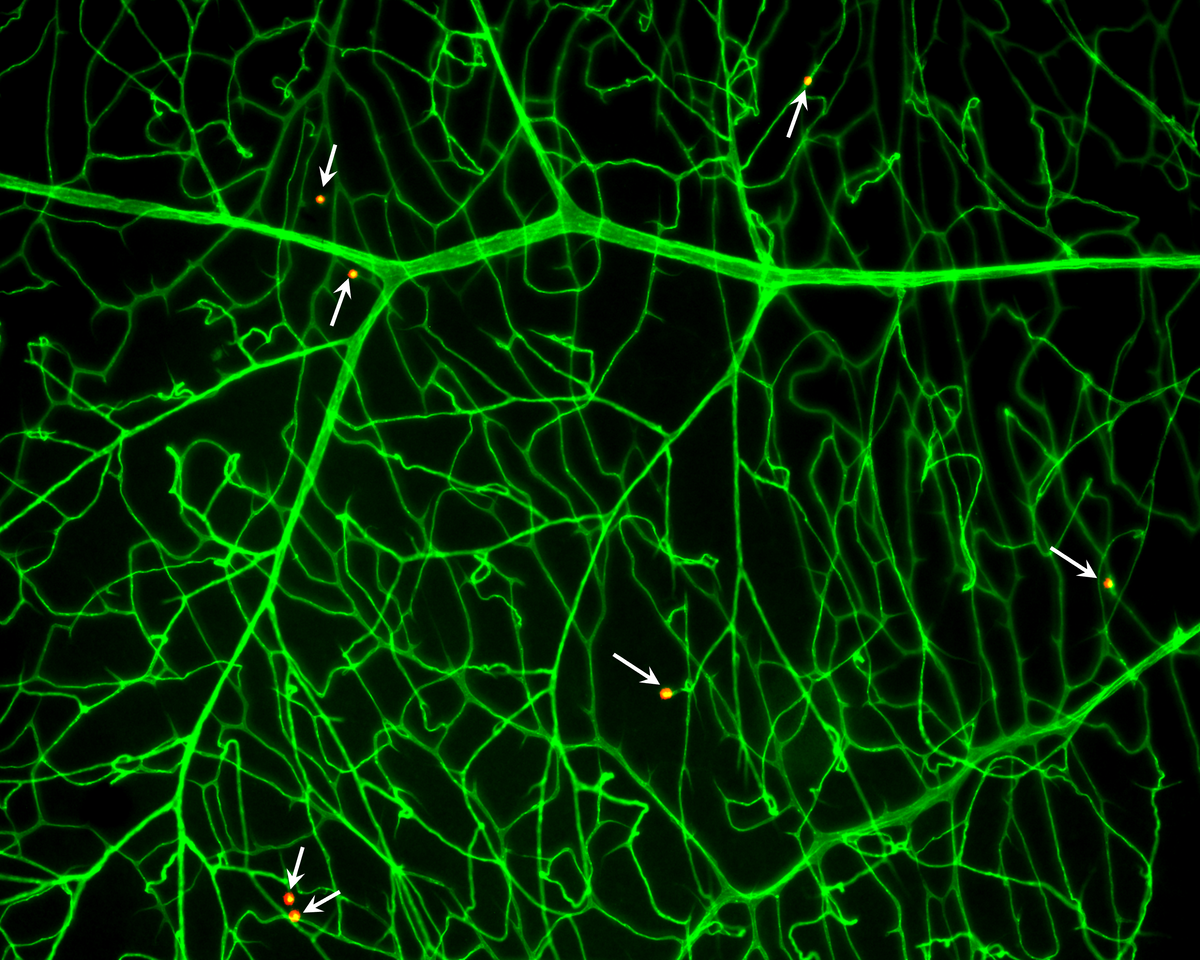
Lessieur studies how neutrophils, the cells that kick off inflammation in the body, contribute to diabetic retinopathy. In particular, she and her colleagues investigate how elastase enzymes that neutrophils release in extracellular vesicles lead to features of diabetic retinopathy.
These elastase-containing vesicles “are like text messages that the cells exchange,” Lessieur explained.
The elastases set off a signaling cascade that promotes increased inflammation. They help the neutrophils kill invading pathogens, but researchers have also observed elevated levels of neutrophil elastase in the blood of people with diabetes compared to people without diabetes (7).
Working in mice with diabetes, Lessieur and her colleagues found that neutrophil elastases trigger inflammation in the eyes and also cause increased blood vessel leakage (8). When the researchers treated these mice with neutrophil elastase inhibitors formulated in eye drops, inflammation receded, and the treatment prevented the degradation of retinal capillaries (9).
Because neutrophils release their elastases in extracellular vesicles, Lesseuir and her colleagues are developing a nanoparticle-based therapeutic to block the release of neutrophil elastase-containing extracellular vesicles and their retinal damaging communication (10).
“We do not want to block all the neutrophil activity,” said Lessieur. “Our goal is to develop a nanotargeted medicine that will block only the disease activated neutrophils.”
A toxic gas turned good
Scientists at Augusta University and Hillhurst Biopharmaceuticals are also targeting retinal inflammation in diabetic retinopathy, but their approach has a gaseous twist. They use low doses of the typically toxic gas carbon monoxide as a new treatment for diabetic vision loss.
“At high doses, carbon monoxide is toxic, but if you step back and think about it, all drugs are toxic at the wrong dose,” said Andrew Gomperts, the cofounder and leader of Hillhurst Biopharmaceuticals. “There's really good data that at low doses, carbon monoxide could be safe enough.”

Carbon monoxide is typically dangerous because it binds to the hemoglobin in red blood cells with a much higher affinity than oxygen — about 240 times higher (11). In high doses, carbon monoxide prevents oxygen from binding to red blood cells, leading to oxygen deprivation and ultimately, death.
In low doses, however, researchers have noticed that carbon monoxide has beneficial effects in a number of diseases (12). In particular, low doses of carbon monoxide can trigger the expression of the gene heme oxygenase 1 (HO-1), which activates anti-inflammatory and anti-oxidative stress pathways.
The team at Hillhurst Biopharmaceuticals are experts in formulating therapeutic gases into liquid treatments so that they are easy for people to take and are formulated in precise dosages. By working with their collaborators at Augusta University led by ophthalmology researcher Pamela Martin, the scientists at Hillhurst Biopharmaceuticals created a low dose carbon monoxide oral drug called HBI-002.
When swallowed, HBI-002 moves through the digestive tract, and the carbon monoxide crosses from the intestines into the blood. It hitches a ride on hemoglobin in the blood and moves throughout the body. When the carbon monoxide gets to the blood vessels in the retina, its presence there induces the expression of the HO-1 gene, leading to a reduction in inflammation in the retina. This leads to an increase in blood flow through the blood vessels in the retina, which reduces the retina’s need to create more blood vessels.
When the researchers gave mice with retinal ischemia HBI-002, the drug stopped any further damage to the retina, and the mice’s visual function improved (13). The research team is excited to test HBI-002’s effectiveness in additional animal models and eventually get the drug into patient hands.
The Hillhurst Biopharmaceutical team is already testing HBI-002 in a clinical study in healthy people to assess its safety and plan to test its effectiveness in treating sickle cell disease soon. “Sickle cell patients also get a retinopathy,” explained Gomperts. “So we’re also excited about that indication along with diabetic retinopathy.”
A chemical fish in a drug ocean
To find new treatments for diabetic retinopathy, scientists at Verseon are diving into a chemical ocean to fish out a cure. Their research team uses artificial intelligence and machine learning to search for overlooked molecules with potential therapeutic effects.
Drug libraries that many research groups screen have about seven to ten million compounds in them at most, but Kita said, “the space of drug-like, synthesizable compounds represents a chemical universe that is estimated to be about 10 to the 33rd power. That’s a billion trillion trillion.”

He added, “Instead of being trapped in this tidal pool, constantly looking in the same space of a few million compounds over and over again for each new target… you can now use a computation to try to explore somewhere like near the continental shelf.”
With Verseon’s molecular modeling engine, the researchers test potential therapeutic molecules against a target protein of known structure in a computer. Rather than look for a new injectable drug for diabetic retinopathy, Kita and his team surveyed the chemical ocean for a drug that people could take orally.
Their lucky catch turned out to be a new class of plasma kallikrein inhibitors (PKIs). When activated in the retina, the plasma kallikrein-kinin (PKK) system sets off a signaling cascade that leads to increased inflammation and greater blood vessel permeability (14).
“One of the nasty things that happens is that when the kallikrein-kinin system gets activated, it will become like a self-fulfilling prophecy of making it worse,” said Kita. But if PKIs could block the runaway train of PKK signaling before it leads to severe blood vessel leakage, they could potentially prevent diabetic retinopathy.
Scientists first got interested in PKIs as a potential treatment for diabetic retinopathy when they noticed high levels of PKKs in eyes of people with the disease compared to healthy ones (15). Studies in rat models of diabetic retinopathy demonstrated that PKIs injected into the eyes reduced inflammation and blood vessel leakiness there (16).
As a class of molecules, PKIs are safe and effective. Regulators have already approved them to treat hereditary angioedema, an inherited condition that causes swelling in multiple tissues — most dangerously in the airway.
When the Verseon scientists gave diabetic rodents one of their newly identified oral PKI candidates, they saw that the treatment decreased blood vessel leakiness in the retina (17). Kita and his team are finalizing the toxicology studies of their PKIs and hope to begin clinical studies soon.
In addition to the research team at Verseon, scientists at the biotechnology companies Rezolute and KalVista are advancing their own oral PKI candidates for diabetic vision loss. KalVista developed an injectable PKI called KVD001 to treat the severe subset of diabetic retinopathy, diabetic macular edema (DME), and they are now developing it into an oral formulation. The Rezolute team is testing their oral PKI candidate for DME in a phase 1b clinical trial.
Most of all, Kita is eager for a cheaper and less invasive treatment for diabetic vision loss for patients.
“When elderly people of a certain age were asked if they would trade extension of life for being able to see even for a year — get their vision back — a very high number of people would prefer to give up years of their lives just to be able to see, especially to see their loved ones,” said Kita. “Vision is a very important thing for us.”
References
- World Health Organization. Diabetes. (2022) https://www.who.int/news-room/fact-sheets/detail/diabetes
- Patel, S. and Sternberg, P. Is There a Cost Benefit to the Ranibizumab Port Delivery System? JAMA Ophthalmol 140, 723-724 (2022).
- Lam, J.D. et al. Identification of RUNX1 as a Mediator of Aberrant Retinal Angiogenesis. Diabetes 66, 1950-1956 (2017).
- Planagumà, J. et al. A Differential Gene Expression Profile Reveals Overexpression of RUNX1/AML1 in Invasive Endometrioid Carcinoma. Cancer Res 64, 8846-8853 (2004).
- Delgado-Tirado, S. et al. Topical delivery of a small molecule RUNX1 transcription factor inhibitor for the treatment of proliferative vitreoretinopathy. Sci Rep 10, 20554 (2020).
- O’Hare, M. et al. Targeting Runt-Related Transcription Factor 1 Prevents Pulmonary Fibrosis and Reduces Expression of Severe Acute Respiratory Syndrome Coronavirus 2 Host Mediators. Am J Pathol 191, 1193-1208 (2021).
- Wang, Y. et al. Increased Neutrophil Elastase and Proteinase 3 and Augmented NETosis Are Closely Associated With β-Cell Autoimmunity in Patients With Type 1 Diabetes. Diabetes 63, 4239-4248 (2014).
- Liu, H. et al. Neutrophil elastase contributes to the pathological vascular permeability characteristic of diabetic retinopathy. Diabetologia 62, 2365-2374 (2019).
- Lessieur, E.M. et al. Neutrophil-Derived Proteases Contribute to the Pathogenesis of Early Diabetic Retinopathy. Invest Ophthalmol Vis Sci 62, 7 (2021).
- Cruz, M.A. et al. Nanomedicine platform for targeting activated neutrophils and neutrophil–platelet complexes using an α1-antitrypsin-derived peptide motif. Nat Nanotechnol 17, 1004-1014 (2022).
- Otto, C.N. 7 - Hemoglobin metabolism. Eds: Elaine M. Keohane, Catherine N. Otto, Jeanine M. Walenga. Rodak's Hematology (Sixth Edition). Elsevier, 91-103 (2020).
- Ryter, S.W. and Choi, A.M.K. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res 167, 7-34 (2016).
- Jadeja, R. et al. HBI-002 treatment improves retinal ischemia-reperfusion injury in mice. Invest Ophthalmol Vis Sci 63, 2983-F0224 (2022).
- Phipps, J.A. and Feener, E.P. The kallikrein–kinin system in diabetic retinopathy: Lessons for the kidney. Kidney International 73, 1114-1119 (2008).
- Gao, B. et al. Characterization of the Vitreous Proteome in Diabetes without Diabetic Retinopathy and Diabetes with Proliferative Diabetic Retinopathy. J Proteome Res 7, 2516-2525 (2008).
- Clermont, A. et al. Plasma Kallikrein Mediates Retinal Vascular Dysfunction and Induces Retinal Thickening in Diabetic Rats. Diabetes 60, 1590-1598 (2011).
- Calton, M.A. et al. An orally dosed plasma kallikrein inhibitor decreases retinal vascular permeability in a rat model of diabetic retinopathy. Invest Ophthalmol Vis Sci 59, 3576 (2018).



