Leber congenital amaurosis (LCA) is one of the most common forms of inherited blindness worldwide. Visual defects resulting from LCA arise from a variety of genetic mutations that block the biochemical cascade of information that begins when light hits the retina (1). Approximately 3,000 people in the United States live with LCA (2).
A group of scientists and ophthalmologists led by Shannon Boye, a neuroscientist at the University of Florida and cofounder of Atsena Therapeutics, recently announced a successful gene therapy intervention that improved night vision in two adult patients with LCA. They published their results in the journal iScience (3).
“If you ask people which of their five senses they would least like to lose, 99.9 percent of people would say their sight,” said Boye.
If you ask people which of their five senses they would least like to lose, 99.9 percent of people would say their sight.
- Shannon Boye, University of Florida
Both patients in the recent study had mutations in the guanylate cyclase 2D (GUCY2D) gene (4). When light hits the eye, sodium and calcium ions flood photoreceptor cells. This sets off a neurochemical cascade that sends visual information to the brain. For this cascade to work, photoceptors must depolarize and turn off. People with GUCY2D mutations have photoreceptors that are perpetually stuck in an on position (5). GUCY2D mutations primarily affect rod cells, the photoreceptors in the eye that function best in low light, so mutations result in poor night vision.
“One special feature of LCA that differentiates it from a lot of other inherited retinal diseases is that these patients, despite their profound deficiencies in vision, typically have very well-preserved retinal structures,” said Kenji Fujita from Atsena Therapeutics.
In the recent clinical trial, surgeons injected the retinas of both patients with an adenovirus vector carrying a wild type copy of the GUCY2D gene. Within days, both patients’ night vision improved when measured using a full field stimulus test (FST). This test requires a patient to peer into a large dome that shines a light from different angles. “The patient literally clicks a button that says, ‘yes, I can see that light,’” said Boye.
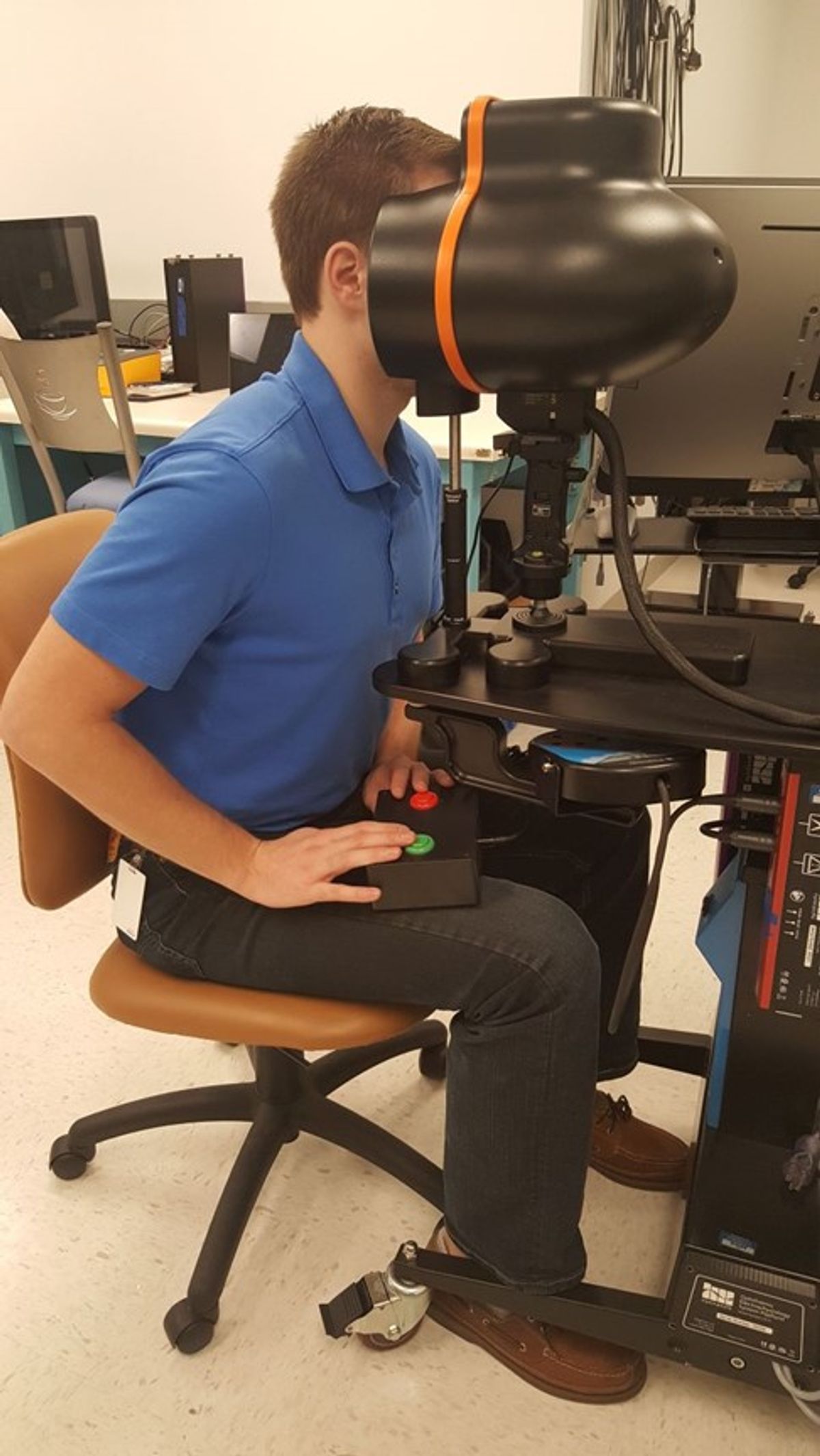
Within eight days of the injection, rod cells in the injection zone within the patients’ eyes that had been inactive began to light up. Visual improvements continued for up to three months after the treatment. This is particularly noteworthy because both patients had been visually impaired since birth. Until now, it was not clear that any treatment for adults with impairments in visual acuity could actually improve vision. Both patients’ pupillary reflexes also improved, and they began detecting objects and people that they couldn’t see before treatment.
“One patient told us after the injection that she was able to see a star for the first time,” said Fujita.
The first ever FDA-approved gene therapy for any condition was Luxturna, which also treated LCA but targeted a different gene called retinal pigment epithelium-specific 65 kDa protein (RPE65). Boye witnessed Luxturna’s success. “I was fortunate to go through graduate school around the time Luxturna was developing. You can't overestimate how important vision is for our ability to navigate life,” she said.
The success of the recent gene therapy for LCA follows in Luxturna’s footsteps. Tomas Aleman, a neuroscientist at the University of Pennsylvania who was not involved in the study, was enthusiastic about the work. “It confirms and solidifies gene therapy as an option for at least a subgroup of patients with these diseases,” he said. “But this is the earliest report of the trial. We'll have to see down the road if this effect will remain for years to come.”
One limitation of the treatment is that it only affects cells in the immediate region of the injection. A smaller volume of liquid is less likely to stress or irritate the eye but might not come into contact with enough eye tissue to affect vision. The amount of volume a surgeon can inject limits the number of photoreceptors that can acquire a functional GUCY2D gene during therapy, so the team is working on an adenovirus vector that can spread beyond the immediate injection site. They hope that this will enable injections at a safe, peripheral site away from the central retinal area, where injections carry the risk of detaching and potentially damaging the retina.
References
- Kumaran, N., Moore, A. T., Weleber, R. G. & Michaelides, M. Leber congenital amaurosis/early-onset severe retinal dystrophy: clinical features, molecular genetics and therapeutic interventions. Br J Ophthalmol 101, 1147–1154 (2017).
- Drack, A. V., Johnston, R. & Stone, E. M. Which Leber congenital amaurosis patients are eligible for gene therapy trials? Journal of American Association for Pediatric Ophthalmology and Strabismus 13, 463–465 (2009).
- Jacobson, S. G. et al. Night vision restored in days after decades of congenital blindness. iScience 25, 105274 (2022).
- Francis, P. J. Genetics of inherited retinal disease. Journal of the Royal Society of Medicine 99, 189–191 (2006).
- Jacobson, S. G. et al. Defining Outcomes for Clinical Trials of Leber Congenital Amaurosis Caused by GUCY2D Mutations. American Journal of Ophthalmology 177, 44–57 (2017).



