With one precise fold on top of another, a butterfly emerges from what was once a single sheet of paper. The traditional art of origami brings paper animals to life from a series of intricate and delicate folds. Inspired by origami’s precision, bioengineers extended its ideas to a medium much more ancient than paper: DNA. In doing so, they created a new way to help the immune system fight off cancer.
DNA origami is like a plug and play kind of platform. You can swap out the antigen to tailor it to different cancers or swap out the antigen to tailor it to infectious disease.
- Yang (Claire) Zeng, Wyss Institute for Biologically Inspired Engineering at Harvard University
Cancer vaccines rally the immune system to kill tumors. By bundling tumor antigens along with adjuvants to increase the immune response, the vaccines activate cytotoxic T cells and amplify other immune pathways to kill tumor cells. With their small sizes and complete programmability, DNA origami nanostructures have emerged as a new and improved cancer vaccine delivery system (2).
DNA origami vaccines are single-stranded pieces of DNA folded onto themselves or held together with short complementary DNA sequences. These DNA strands can self assemble into an elegant three-dimensional nanostructure. Using computer programs, scientists design DNA origami structures with precise folds and often attach molecules onto them — like vaccine antigens and adjuvants — in specific numbers and arrangements. Due to their small size, immune cells easily take up these origami nanostructures, making them an ideal vaccine delivery vessel.
“DNA origami is like a plug and play kind of platform,” said Yang (Claire) Zeng, a cancer immunologist and bioengineer at the Wyss Institute for Biologically Inspired Engineering at Harvard University. “You can swap out the antigen to tailor it to different cancers or swap out the antigen to tailor it to infectious disease.”

A barrel-shaped pH surprise
Delivering cargo to the right immune cells at the right times has been one of the major challenges facing cancer vaccines. With some clever engineering, Baoquan Ding, a bioengineer at the National Center for Nanoscience and Technology, and his team designed a solution in the shape of a rigatoni with a surprise inside (3).
Using mice, Ding and his team injected their DNA origami vaccine under the skin. The nanostructures trafficked to the lymph nodes, sites teeming with dendritic cells ready to take up the vaccine. In the lymph nodes, “the cells of the immune system will basically swallow this structure” into an endosome, he said.
With the more acidic pH inside the endosome compared to the cellular environment outside, Ding’s tubular DNA origami structure splits lengthwise along its seam and flattens into a rectangular sheet. This deliberate transformation of the DNA origami carrier reveals the cancer vaccine components precisely when and where they’re needed: inside the immune cells. In its flat, activated orientation, the tumor-specific antigen and two different adjuvants enhance the immune cell’s response to tumor cells.
It's quite powerful technology, but it's still in an early stage.
– Baoquan Ding, National Center for Nanoscience & Technology
Some vaccines require lipid nanoparticles (LNPs) to deliver their vaccine components into dendritic cells, such as the SARS-CoV-2 vaccines produced by Moderna and Pfizer-BioNTech, but LNPs are never exactly the same size.
“If their size is around 100 nanometers, they are always minus or plus 30 nanometers. Their size is not precise,” Ding said. Because of this, “you don't know how many mRNA molecules are inside the particle.”
For DNA origami vaccines, though, “the pore size, shape, geometry, and molecular weight are very precise — down to the single base level. And then we can precisely control the number of adjuvants and then antigens encapsulated inside our origami structures,” he added. This precision allows the cells that take up the vaccine to produce a consistent immune response.
When Ding and his team tested how well their pH-activated DNA origami vaccine treated mice with melanoma or carcinoma, they found that the mice given the vaccine survived longer than those not given the vaccine, and their tumors grew much more slowly. The vaccine also inhibited metastasis and recurrence of their tumors.
Ding and his team are eager to collaborate with other groups to test their DNA origami vaccine in nonhuman primates and eventually in clinical trials.
“It's quite powerful technology, but it's still in an early stage,” said Ding. “There's a great potential.”
Not just DNA, but RNA too

DNA is not the only genetic material to fold into tiny intricate structures; RNA can too. In fact, because the human body often recognizes RNA as a threat due to the many viruses that use RNA as their genetic material, the RNA that makes up the origami vaccine can act as its own adjuvant, amplifying the immune response by itself.
Considering these advantages of an RNA based platform, bioengineer Hao Yan and cancer immunologist Yung Chang at Arizona State University designed an RNA origami vaccine composed of a single strand of RNA that self assembles into a rectangular nanostructure (4). Because they can make the RNA origami out of a single strand of RNA, the research team can decrease the time and cost of synthesizing this structure compared to other nucleotide-based origamis that require many short support strands of DNA or RNA to hold together. Yan and his colleagues have previously constructed both DNA and RNA origami into all kinds of shapes, including a rhombus, rectangle, and heart (5).
Unlike RNA in its typical linear state, when Chang and Yan folded it, their RNA origami nanostructure was surprisingly stable. The RNA origami structure resisted damage from RNA digesting enzymes in blood plasma and serum that degraded the same unfolded RNA sequence.
“It's a nanostructure that can be actually kept in the refrigerator for more than a year,” said Chang. “Because of the stability, you don't need all those lipid nanoparticles to stabilize it, so that actually significantly reduces the cost of manufacturing.”
Using a mouse model of colon cancer, Yan and Chang reported that their RNA origami vaccine prevented tumor formation and shrunk existing tumors, depending on when the scientists administered the vaccine. The researchers then rechallenged mice that had received the RNA origami vaccine with additional colon cancer cells, but their tumors never grew, indicating that they had developed immunity to the cancer.

Chang and Yan are now working on attaching a tumor-specific antigen to their RNA origami nanostructure and are testing it in a melanoma mouse model. They are eager to test their RNA origami vaccine in other models and are working on spinning out a company to develop their vaccine further.
“We actually have very convincing data that this platform works well, and also cost effectively, compared to the conventional platform,” said Chang. “I am very optimistic about this vaccine platform.”
Precise nanoscale spacing
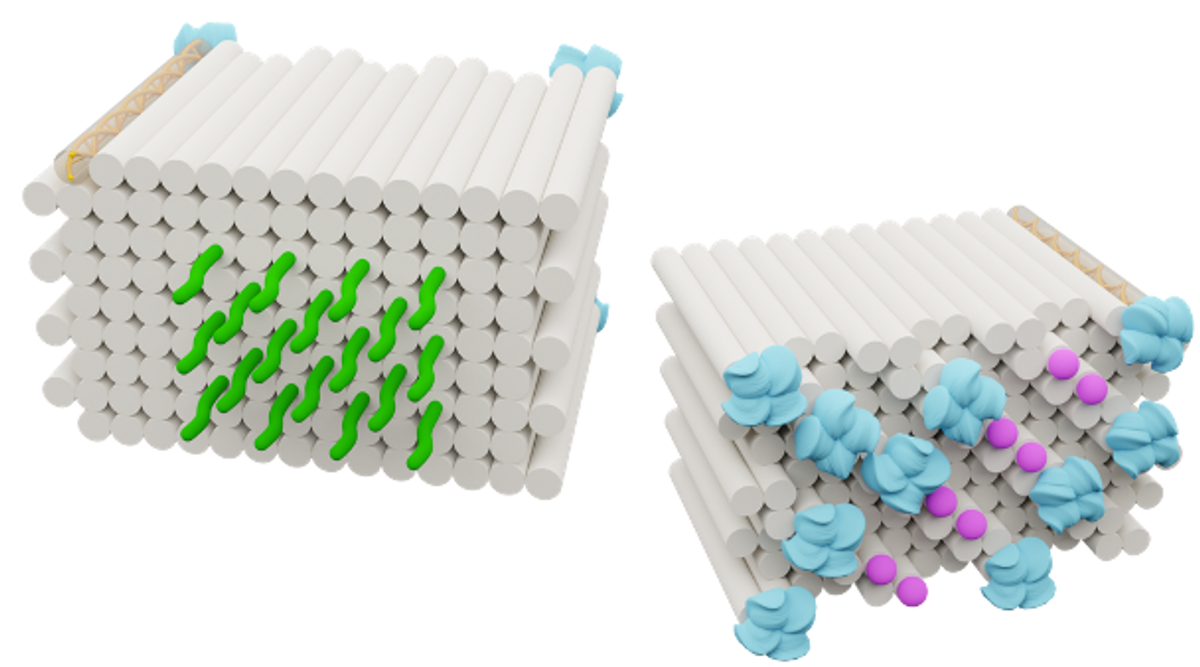
Like ice cubes decorated with tiny strings, Zeng's DNA origami vaccine, dubbed DoriVac, shows just how important precision is when it comes to making an effective cancer vaccine.
The common vaccine adjuvant CpG oligodeoxynucleotide (CpG) binds to the TLR9 receptor inside dendritic cells to amplify the immune response to vaccination. But different densities of CpG molecules presented on a vaccine activate diverse immune responses (6). A high CpG density leads to a cellular immune response — optimal for cancer immunotherapy — and a low CpG density activates a humoral response.
For most vaccines, “when they’re delivered through other nanoparticles, in that case, it’s just an average nano spacing. It’s not precise nano spacing,” said Zeng. This imprecise CpG spacing can lead to a mix of different immune responses, which decreases the cancer vaccine’s effectiveness. “Using DNA origami, we can offer precise nanoscale spacing and investigate whether the spacing is the right spacing to induce the immune response that we want that can kill the tumor cells.”
In a recent preprint, Zeng and her colleagues described multiple different square-block DNA origami vaccines where they attached different tumor antigens on one side and CpG adjuvants arranged in precise spacings with either 2.5 nm, 3.5 nm, 5 nm, or 7 nm between the individual CpG molecules (7).
They found that CpG molecules arranged 3.5 nm apart on their DNA origami vaccine triggered the best immune response both in vitro and in a mouse model of melanoma. When the researchers gave the mice the optimized DNA origami vaccine and then treated them with melanoma cancer cells, only one mouse that had received the vaccine ever developed a tumor. When used to treat an already established tumor, this DNA origami vaccine prolonged mouse survival and slowed tumor growth.
Most striking, when Zeng and her team treated mice with aggressive melanoma or lymphoma with their DNA origami vaccine and the immune checkpoint inhibitor anti-PD-L1, they discovered that the combined effect had an even stronger anticancer response. In the melanoma group, four out of the five mice tested survived, and in the lymphoma group, all of the mice survived.
“All those elements coming together can really reverse the repressive tumor microenvironment and make other immunotherapies, such as the immune checkpoint inhibitor, more responsive,” said Zeng.
She and her team are now testing the effectiveness of their DNA origami vaccine in humanized mouse models and plan to perform toxicity and safety testing soon. Zeng plans to spin out a company based on this technology sometime next summer.
“We already hope that DNA origami nanoparticles could be the next generation of vaccine,” she said.
References
- Ventola, C.L. et al. Cancer Immunotherapy, Part 3: Challenges and Future Trends. P&T 42, 514-521 (2017).
- Pitikultham, P. et al. Stimuli-Responsive DNA Origami Nanodevices and Their Biological Applications. ChemMedChem 17, e202100635 (2022).
- Liu, S. et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat Mater 20, 421-430 (2021).
- Qi, X. et al. RNA Origami Nanostructures for Potent and Safe Anticancer Immunotherapy. ACS Nano 14, 4727-4740 (2020).
- Han, D. et al. Single-stranded DNA and RNA origami. Science 358, eaao2648 (2017).
- Leleux, J.A. et al. Biophysical Attributes of CpG Presentation Control TLR9 Signaling to Differentially Polarize Systemic Immune Responses. Cell Rep 18, 700-710 (2017).
- Zeng, Y.C. et al. Optimizing CpG spatial distribution with DNA origami for Th1-polarized therapeutic vaccination. Preprint at: https://www.biorxiv.org/content/10.1101/2022.06.08.495340v2.abstract



