After years of watching children with cancer and immune disorders in the pediatric intensive care unit suffer from sometimes fatal respiratory illnesses after receiving stem cell transplants, Matt Zinter, a clinical researcher at the University of California, San Francisco, decided to act.
He wanted to know what made some sick children more susceptible to respiratory problems following stem cell transplants. He suspected that children with poor lung function were more likely to have bad outcomes. Previous work showed that patients with weak lungs were more likely to die following hematopoietic cell transplantations (HCT), a stem cell therapy used to treat blood cancers like leukemia.

Zinter and his team recruited 104 patients scheduled to receive an HCT to determine if there was something different about the function and environment of the lungs of children who developed respiratory illnesses after receiving their transplants compared to kids who did not. They tested every patient’s lung function and collected liquid samples from the bronchioles via a somewhat invasive procedure known as bronchoalveolar lavage (BAL).
The researchers reported in Science Translational Medicine that patients with weakened lung function had altered lung microbiomes and were more likely to have complications following HCT, although Zinter warned that the association is correlative (1). Andrew Koh, a microbiome researcher from the University of Texas Southwestern Medical Center who was not involved in this research, is cautiously excited about the “intriguing findings.”
“The problem with the lung microbiome field is that a lot of people studying the oral microbiome collect sputum. But that’s not really down in the lung. The only way to do that is what these guys did, which is to do an invasive procedure by doing a BAL and finding the bugs that are associated in the lower bronchi. That’s not trivial,” said Koh. “These guys made a Herculean effort doing these BALs. That's really above and beyond.”
“They were smart and savvy,” added Koh. “They’re very cautious about not overstating their claims because they realized that this is tricky business.”
During a BAL, a clinician inserts a bronchoscope deep into the lungs to collect fluid from the furthest point in the respiratory system where oxygen is delivered to the blood, the alveoli. Although this procedure is considered minimally invasive, it requires anesthesia. All 104 patients in Zinter’s study had a disease qualifying them for HCT, but only half of the 104 did not have overt symptoms of respiratory problems based on a series of respiratory tests that measured lung capacity and respiration. “Typically, when you’re going to do a BAL, it’s when somebody is really freaking sick, and you’re trying to figure out what’s going on,” said Koh.
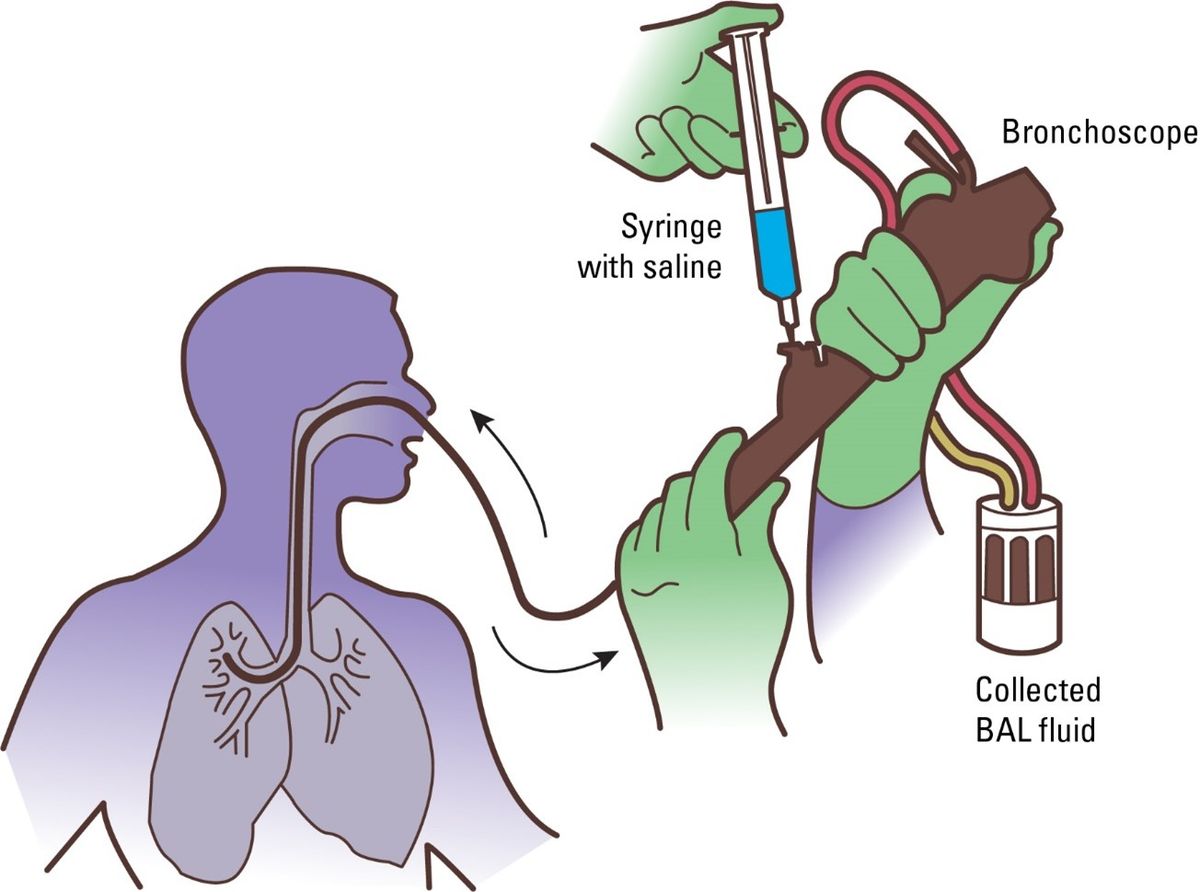
Zinter and his team analyzed the microbial transcriptomes of samples collected from the BAL to determine if there were any differences between the microbial makeup of patients with normal and altered lung function. They discovered that patients with worse lung function and higher post-HCT mortality rates had depleted levels of microbes commonly found in the respiratory system and high levels of bacteria normally found on the skin or in the nose such as Staphylococcus.
“During stem cell transplants, the immune system is very compromised, and the patient is at risk of infection…This study suggests that maybe having a depleted microbiome means you’ve lost some level of protection,” said Zinter. “As the immune system redevelops after the [HCT], it has to learn what to protect and what to attack. Part of that learning process is learning how to recognize good from bad. I wonder if the lack of a microbiome in the lungs could limit how well the immune system can develop.”
Zinter may need to use an animal model to answer these questions and ultimately to determine if these changes in the microbiome are correlative or causative. Gut microbiome researchers often use reverse translation to recreate the microbial makeup of the human gut in disease to see how it affects a mouse. Koh thinks that Zinter could recreate the altered lung microbiome he identified in mice to see if it causes changes in respiratory function and HCT outcome.
“These guys did a ton of work already. This is kind of the first building block in the foundation so that we can get to the next level,” said Koh.
Reference
Zinter, M.S. et al. Pulmonary microbiome and gene expression signatures differentiate lung function in pediatric hematopoietic cell transplant candidates. Sci Transl Med 14 (2022).



